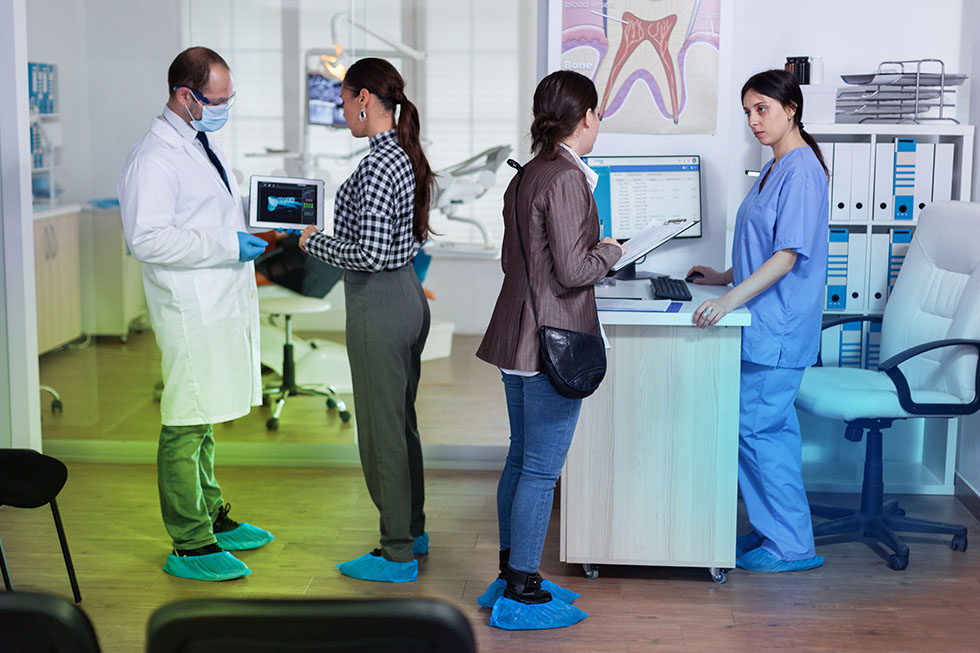
Clinical Trial Services
Over the last 15 Years we made an impact that is strong in pharma industry.
Cito’s Clinical Development Services comprises of various services such as Bioavailability/Bioequivalence studies, Clinical Trials and allied supporting services for the pharmaceutical industry for their submission to regulated and semi regulated market. Having a basket of associates, Cito is uniquely structured to support the needs of healthcare industry during all stages of clinical development, whether be a complete bioequivalence study or a clinical trial management program. We are capable to provide you assured quality to answer your clinical need.
We organize for our associates various pre-clinical and Clinical Development Services.
Explore All Cito Healthcare Services ? Click here
Pre-clinical
Expected studies on toxicity and others on small animals, large animals both in GLP and non-GLP environments.
Early Stages
Phase-I for those products manufactured in India Phase-I from Second cohort on-wards for those products manufactured outside of India. BA/BE studies on both healthy and patient populations
- Acute
- SubAcute
- Sub Chronic And Cronic
- Reproductive And Development Toxicity
- Genetictox
- Immunotoxicology
- Efficacy Models
- Physical Chemistry
- Ecotoxicity
- Infectious Models
- Routes Of Administration
- Biological Tests
- Dose Linearity Studies including dose escalations
- Multiple Dose and Steady State
- Fasting
- Food Effect
- Drug Drug Interactions
- Design & Implementation of Clinical Phases
- Clinical Trial Operations (Site Initiation, Monitoring and Closeout)
- Investigator Site Selection
- -- Clinical Trial Supplies Management
- Site Management
- Analysis & Interpretation of Healthy subjects & Patients studies
- -- Project Management (Start to End)
- -- Clinical Safety (PSUR and safety Reports)
- -- Clinical Study Monitoring, Auditing & Training
- Regulatory Consulting